A good introduction to biomedical near infrared imaging is found here, while a more technical survey is available here. Both pages are from the Biomedical Optics Research Group at the University of London. The descriptions are given at the level of a good high school biology course. The specific application of the first article is human brain tissue studies, although the discussion is basic enough to yield insight into near infrared medical imaging in general. Fundamentally, the basic concepts are that near infrared spectroscopy is limited by the absorption wavelengths of some predominant compounds in the body; in this case, the large absorption peak at 650nm from oxygenated haemoglobin (HbO2, corresponding to oxygenated blood) and that at 1000nm from water set the infrared window. Within the window are chromophores: compounds that demonstrate absorption peaks within the extrema. In this case, certain bonds in cytochrome c oxidase (CtOx), an enzyme governing oxygen uptake in mitochondria (the "power plant" of the cell), show a broad peak near 830nm.
This last fact highlights a fundamental assumption in near infrared imaging: it seems that in many medical applications, the physical parameter measured is oxygenation or, more generally, metabolic contrast. Thus, in the brain tissue experiment, changes in the near infrared measurements near 830nm can be linked through CtOx to alterations in the oxygen acceptance rate; this increased oxygenation is assumed correlated to augmented brain tissue activity. Many medical imaging techniques make similar assumptions of oxygenation and activity, although in other fields (such as oncology) the correlation is more direct. Finally, the site also details modeling optical paths in tissue, including scattering at the entrance interface and modified Beer-Lambert absorption calculations through the medium.
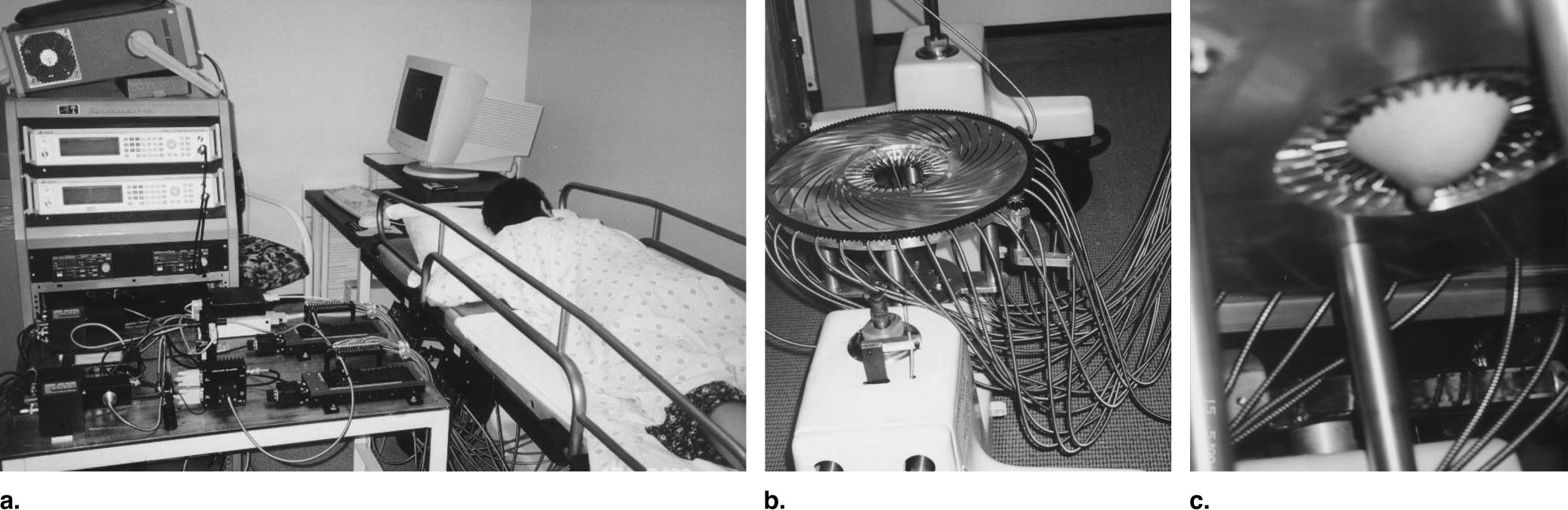
As mentioned, the detection of increased metabolism is especially relevant in cancer studies. A large application is in breast cancer detection where the non-ionizing aspect of optical methods points to a clear advantage over more traditional x-ray mammography. The availability of imagers in the near infrared appears to have pushed recent research interests. Since increased oxygenation is correlated to cancerous growths (due to increased vascularization and organelle population), and since good chromophores exist which indicate oxygenation and metabolism, near infrared imaging provides a good window for breast cancer imaging. This article from the journal Breast Cancer Research was found to provide a good review of this field, including background history and older techniques. Much of the field seems to concentrate on image reconstruction techniques; the article mentioned focuses on diffuse optical tomography (DOT), a method for reconstructing three-dimensional profiles from the acquired images. The sample images below were taken from a more recent article detailing clinical accuracy of the modality. An intensity-modulated laser at 785nm distributed into a circular array of sources and detectors acquires cross-sectional images of the perpendicular breast:

These cross sections can then be processed and stacked to produce the final image:
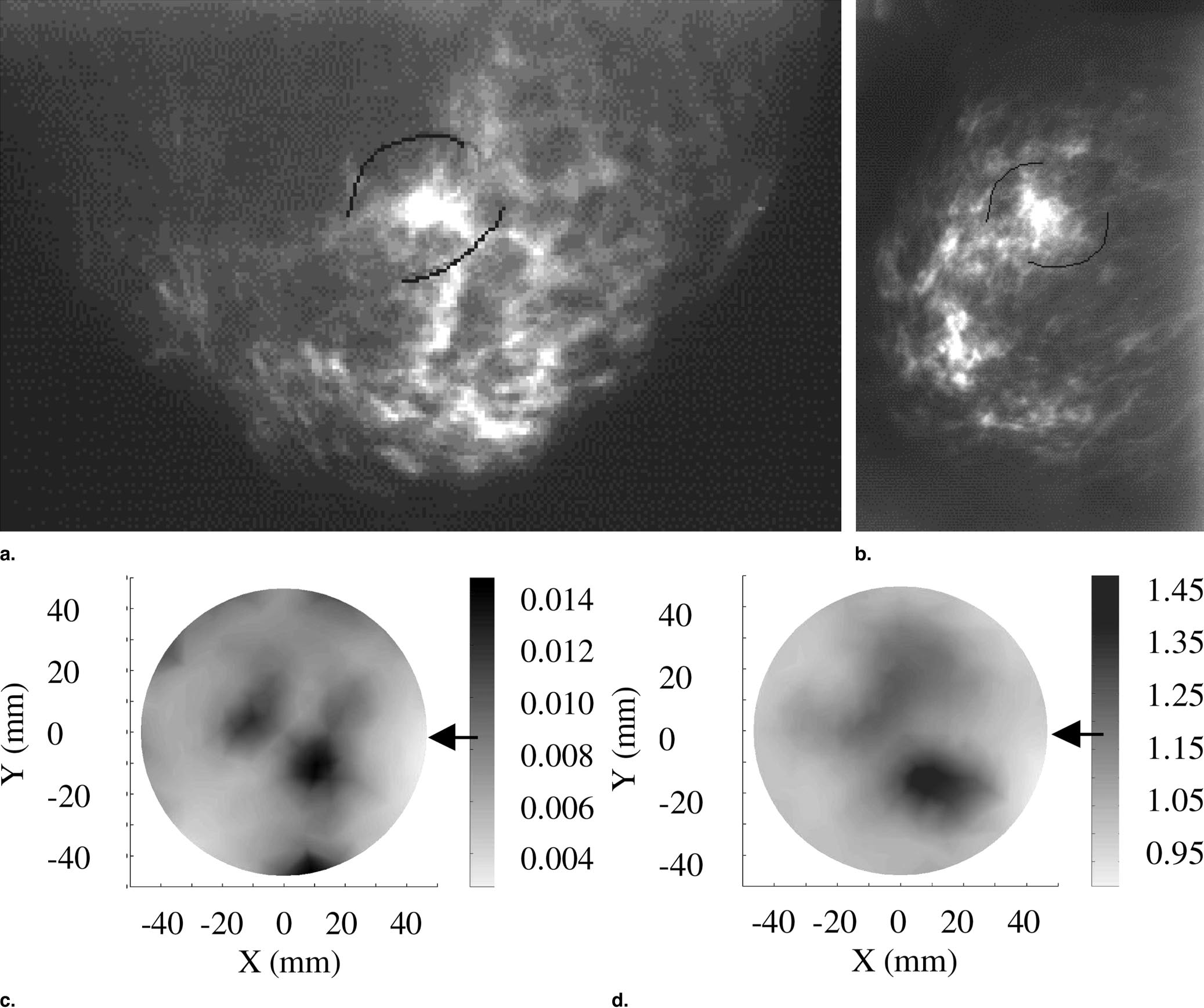
Finally, an interesting biomedical application of reflective near infrared imaging is detailed here. A combination of visual spectrum and near infrared imaging is used to locate plaque inside arteries in vivo. The object is imaged at different wavelengths with different lights, the results are then calibrated and processed to produce a probability density contour map of the object which identifies likely regions of plaque build up. Below, the left image shows the object, an exposed carotid bifurcation, imaged under normal light conditions. At right is shown the contour map constructed from near infrared images. The white box shows a region of plaque within the artery.

Home Introduction Applications Methodology Modeling Results Conclusion References Appendix I Appendix II